1) To understand how the molecular structure of a specific compound affects its photonic properties. By understanding the influence of molecular structure on photonic properties one can achieve a better understanding of how certain molecules obtain their photonic properties.
2)Conduct synthesis of fluorescent dyes and understanding the implications of altering their structure. Conducting experiments in the lab and visually seeing the fluorescent properties of molecules creates a better appreciation and understanding of the photonic properties.
3) Understand how molecular structure and intracellular targeting of the flurophore are related
The first two objectives have been currently explored in the past two weeks. However, before discussing these concepts one must first understand basic terminology and aspects of organic chemistry.
Chromophore: A chromophore is technically any part of the molecule (functional group) that is responsible for its color.
The definition above does describe a chromophore, however the concept of “color” is relative to the observer. Humans for example define color as the wavelengths in the visible light region of the electromagnetic spectrum (380nm – 750 nm). Other animals such as Bee’s or snakes can see into the ultraviolet and infrared region. However, humans can detect the ultraviolet region though their senses of touch – We can detect how much infrared radiation is being emitted by feeling a burning sensation. In essence chromophores are able to absorb and reflect certain wavelengths of visible light region. With all of this in mind we can now broaden our definition of chromophore:
Chromophore: A chemical group capable of selective light absorption resulting in the coloration of certain organic compounds.
| Organic Objective-1 |
Now that we understand the basics behind a chromophore we can now try to understand how the molecular structure of a specific compound affects its photonic properties. One major property that makes a molecular absorb light in the visible region is conjugated-pi systems, or alternating single and double bonds as seen in the molecule below:

When a photon/energy is absorbed by this molecular the double bonds can move about the molecule from one end to the other creating color. Although a conjugated pi system is essential for organic dyes other aspects of molecular structure play a vital role in their overall photonic properties, which will be explored more extensively in upcoming posts. (Look for posting entitled Organic Lego’s)
|Organic Objective 2 |
Physically synthesizing and handling organic dyes can give one a better appreciation and understanding of the chemistry behind these molecules. However, understanding the mechanistic detail of the reactions is not necessary to explore their photonic properties in the laboratory. The photonic properties of these molecules can be seen as soon as the reagents are mixed together. Additionally, the use of thin layer chromatography (TLC) can give a quantitative perspective of the reaction. The reaction below was conducted in the lab:
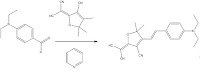
The reagents in the above reaction were placed in a flask and stirred at room temperature for about 48 hours. After 48 hours the water was added to the reaction to solidify the final product above (the final product is conveniently insoluble in water). After obtaining the final product TLC was performed with the final product and starting reagents.
The final product was a dark red dye and can be seen below in a 3D representation (makes the blog look sweet!):

Since we understand how molecular structure influences photonic properties we can explore the reasons for why the molecule synthesized above exhibits a dark red color. Looking at the molecular structure above it is obvious that the final product has a pi-conjugated system which is responsible for its photonic properties. However, other factors such as position on the ring, lone pair electrons, and electron withdrawing/accepting groups affect the photonic properties of molecules. In the upcoming Organic post entitled “Organic Lego’s” these factors along with the synthesis of another organic dye will be explored.















No comments:
Post a Comment